What Is The Liquid Inside The Thermometer
Alright, let's talk thermometers. We all know what they do – tell us the temperature. But what about that liquid inside? What is that stuff, and why does it work the way it does? You might be surprised to learn there's more to it than just "red liquid." We'll break down the types, the physics, and even touch on the history a bit.
Different Liquids, Different Thermometers
Back in the day, you probably remember seeing mercury thermometers everywhere. Today, you're more likely to find ones with alcohol or a similar liquid. Why the change?
Mercury Thermometers: The Classic (and Controversial) Choice
For a long time, mercury was the king of thermometer liquids. It's a metal that's liquid at room temperature, with a pretty consistent rate of expansion. That means for every degree Celsius (or Fahrenheit) the temperature rises, the mercury expands by a predictable amount. This predictability is key for accurate readings. Mercury also has a high boiling point (around 356°C or 673°F) and a low freezing point (-39°C or -38°F), giving it a wide usable range. This allowed for thermometers that could be used in a variety of applications and climates.
However, mercury is also a potent neurotoxin. If a mercury thermometer breaks, the spilled mercury can be hazardous, especially to children and pregnant women. This is why you rarely see them anymore, and their use is heavily regulated.
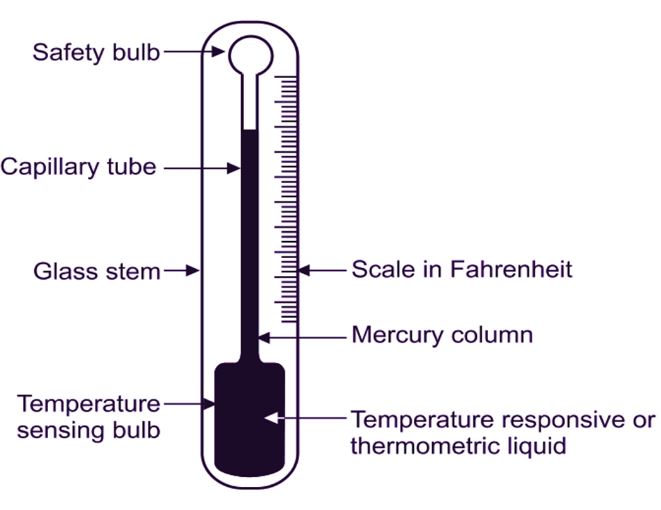
The way it works is pretty straightforward. The bulb at the bottom of the thermometer contains the mercury reservoir. As the temperature rises, the mercury expands. This expansion forces the mercury up the narrow capillary tube – that thin glass tube you see inside. The height of the mercury column corresponds to a specific temperature reading on the scale. The narrower the capillary, the more sensitive the thermometer is, as a small expansion results in a greater change in height.
Alcohol Thermometers: The Modern Alternative
These days, most thermometers use alcohol (usually ethanol) or other similar organic liquids. These are often dyed red or blue to make them easier to see. These are a much safer alternative to mercury and far less expensive.
Like mercury, alcohol expands as temperature increases. However, its coefficient of expansion (the amount it expands per degree) is different. This means the thermometer needs to be calibrated differently. Typically, alcohol-filled thermometers are less accurate than mercury thermometers over a wide temperature range. However, modern manufacturing techniques have improved their accuracy significantly.
A key advantage of alcohol is its lower freezing point (typically around -114°C or -173°F). This makes them suitable for measuring very low temperatures, something mercury thermometers struggle with. However, alcohol also has a much lower boiling point (around 78°C or 172°F), limiting their usefulness at higher temperatures. The useful range is less than that of mercury.
Beyond Mercury and Alcohol: Other Options
While mercury and alcohol are the most common, other liquids are sometimes used in specialized thermometers. These include:
- Pentane: Used for very low temperature applications due to its extremely low freezing point.
- Toluene: Another organic liquid with a relatively low freezing point and a higher boiling point than alcohol, making it suitable for a wider range of temperatures.
The Physics Behind It: Thermal Expansion
The fundamental principle behind all these liquid-in-glass thermometers is thermal expansion. This is the tendency of matter to change in volume in response to changes in temperature. When a substance is heated, its particles move more and thus maintain a greater average separation. Because thermometers are sealed, the liquid can only expand along the calibrated glass tube. This expansion is directly proportional to the temperature change.
The amount of expansion is described by the coefficient of thermal expansion, which is different for each substance. This value tells you how much the substance will expand for each degree Celsius (or Fahrenheit) increase in temperature. A higher coefficient of expansion means the substance will expand more for the same temperature change.
Let's break it down using a bit of basic physics. The change in volume (ΔV) is related to the original volume (V), the coefficient of thermal expansion (α), and the change in temperature (ΔT) by the following equation:
ΔV = V * α * ΔT
This equation highlights why the coefficient of thermal expansion is so important for thermometers. A liquid with a higher coefficient of expansion will show a greater change in volume (and thus a larger movement up the capillary tube) for the same temperature change, making the thermometer more sensitive.
Factors Affecting Thermometer Accuracy
While the choice of liquid is crucial, several other factors influence thermometer accuracy. These include:
- Calibration: Thermometers must be accurately calibrated against a known standard to ensure correct readings. This involves marking the scale on the glass tube to correspond to specific temperatures, such as the freezing and boiling points of water.
- Glass Quality: The glass used for the thermometer must be of consistent quality and have a low coefficient of thermal expansion itself. Otherwise, the glass itself could expand or contract with temperature changes, affecting the accuracy of the liquid's readings.
- Capillary Diameter: A narrower capillary tube makes the thermometer more sensitive, but it also increases the risk of errors due to surface tension effects.
- Immersion Depth: Some thermometers are designed to be partially or fully immersed in the substance being measured. Incorrect immersion can lead to inaccurate readings because the thermometer is not at the same temperature as the substance.
Modern Thermometers: Beyond Liquids
While liquid-in-glass thermometers are still common, especially for basic applications, electronic thermometers are becoming increasingly prevalent. These thermometers use devices like thermocouples or thermistors to measure temperature. Thermocouples generate a voltage that changes with temperature, while thermistors change their electrical resistance. These changes are then measured by electronic circuits to determine the temperature.
Electronic thermometers offer several advantages over liquid-in-glass thermometers, including higher accuracy, faster response times, and the ability to measure a wider range of temperatures. They are also less susceptible to errors due to parallax (the apparent shift in the position of an object when viewed from different angles). And, crucially, they don't contain hazardous liquids that could be released if the thermometer breaks.
So, the next time you look at a thermometer, you'll know a bit more about the science behind it. Whether it's classic mercury, safe alcohol, or advanced electronics, they all rely on fundamental physics principles to give us accurate temperature readings.
