What Type Of Current Do Batteries Produce
Batteries, the unsung heroes of our portable world, silently power everything from our smartphones and laptops to electric vehicles and emergency flashlights. But have you ever stopped to consider the specific type of electrical current they provide? The answer, while seemingly simple, involves a fascinating interplay of electrochemistry and electron flow. The short answer is that batteries produce direct current (DC), but let's delve into why and how.
Understanding Direct Current (DC)
Direct current, as the name suggests, is an electrical current that flows in one direction. Think of it like a river flowing downstream. The electrons, the negatively charged particles that constitute electrical current, consistently move from the negative terminal of the battery to the positive terminal through the connected circuit. This unidirectional flow is the defining characteristic of DC.
DC is characterized by a constant voltage polarity. This means that the positive terminal of the battery remains positive, and the negative terminal remains negative. This is crucial for many electronic devices that are designed to operate with a specific voltage polarity; reversing the polarity could damage or destroy them. Imagine trying to force water uphill; it simply won't work in the intended manner. Similarly, forcing current in the opposite polarity of what a device is engineered for can lead to component failure.
Examples of devices that rely heavily on DC power include:
- Electronic Devices: Smartphones, laptops, tablets, and most other portable electronics rely on DC power from their internal batteries.
- LEDs: Light Emitting Diodes (LEDs) require DC current to function.
- Electric Vehicles: While electric vehicles use AC motors for propulsion (more on this later), the battery pack itself delivers DC power. This DC power is then converted to AC by an inverter.
- DC Motors: Many small motors, like those found in toys and appliances, are specifically designed to run on DC power.
The Electrochemical Basis of DC in Batteries
The magic behind a battery's ability to generate DC lies in its internal chemical reactions. A battery is essentially an electrochemical cell that converts chemical energy into electrical energy. Let's break down how this happens, using a generic battery as an example:
Components of a Battery
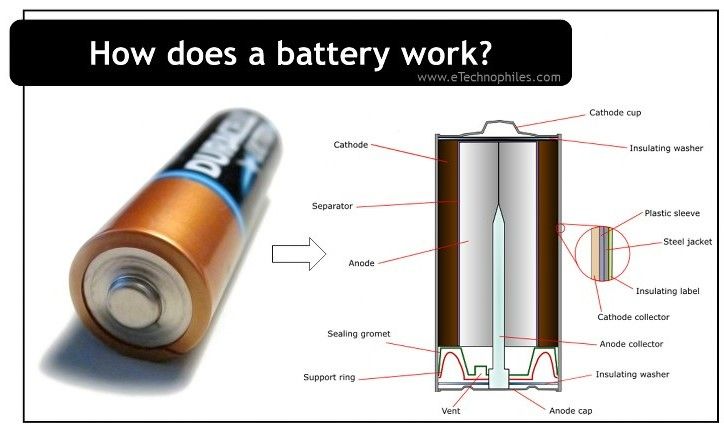
A typical battery consists of three key components:
- Anode (Negative Electrode): The anode is the electrode where oxidation occurs. Oxidation is the process where a material loses electrons. In a battery, the anode is made of a material that readily releases electrons. For example, in a typical alkaline battery, the anode is made of zinc.
- Cathode (Positive Electrode): The cathode is the electrode where reduction occurs. Reduction is the process where a material gains electrons. In a battery, the cathode is made of a material that readily accepts electrons. For example, in an alkaline battery, the cathode is made of manganese dioxide.
- Electrolyte: The electrolyte is a substance that allows ions to move between the anode and the cathode. Ions are atoms or molecules that have gained or lost electrons, giving them an electrical charge. The electrolyte facilitates the chemical reactions that generate electron flow. For example, in an alkaline battery, the electrolyte is potassium hydroxide.
The Electrochemical Process
The core process involves the following:
- Oxidation at the Anode: The anode material undergoes oxidation, releasing electrons. These electrons accumulate at the negative terminal of the battery. The chemical reaction generates ions, which travel through the electrolyte.
- Ion Transport through the Electrolyte: The ions produced at the anode migrate through the electrolyte towards the cathode. This completes the internal circuit within the battery.
- Reduction at the Cathode: At the cathode, the cathode material undergoes reduction, accepting the electrons that have traveled through the external circuit. This consumption of electrons neutralizes the ions arriving from the electrolyte.
- Electron Flow through the External Circuit: When an external circuit is connected between the anode and cathode, the electrons released at the anode flow through the circuit to the cathode, powering any devices connected in the circuit. This continuous flow of electrons constitutes the DC current.
This process continues until the reactants at the anode or cathode are depleted, at which point the battery is "dead." In rechargeable batteries, the process can be reversed by applying an external voltage, replenishing the reactants and allowing the battery to be used again. It's crucial to remember that the chemical reactions drive the electron flow, ensuring that the current always flows in one direction.
Why Batteries Produce DC, Not AC
The fundamental reason batteries produce DC, and not alternating current (AC), stems directly from the nature of the electrochemical reactions within. The oxidation and reduction reactions happen in a consistent manner, with electrons flowing from the anode to the cathode. There is no inherent mechanism within a standard battery to periodically reverse the direction of electron flow, which is the defining characteristic of AC.
AC, on the other hand, involves electrons flowing back and forth, periodically changing direction. This change in direction is driven by an alternating voltage polarity. Devices like generators and alternators, which rely on electromagnetic induction, are specifically designed to produce AC. These devices typically involve rotating magnets and coils of wire, creating a changing magnetic field that induces an alternating voltage and current.
Consider this analogy: think of a battery as a one-way water pump. It consistently pumps water (electrons) in one direction. An AC source, like a generator, is more like a reciprocating pump that pushes and pulls water (electrons) back and forth.
DC to AC Conversion
While batteries inherently produce DC, it is possible to convert DC to AC using a device called an inverter. Inverters use electronic switching circuits to rapidly switch the polarity of the DC voltage, creating an alternating current waveform. This is essential in applications where devices require AC power but only DC power is available from batteries. For example, electric vehicles utilize inverters to convert the DC voltage from the battery pack into AC voltage to power the electric motor.
The process of converting DC to AC involves sophisticated electronics, and is very different from the fundamental operation of a battery.
Conclusion
In summary, batteries produce direct current (DC) because the electrochemical reactions within the battery drive a consistent, unidirectional flow of electrons from the anode to the cathode. The constant voltage polarity ensures that the current flows in only one direction, making batteries ideal for powering a wide range of electronic devices and systems. While DC can be converted to AC using inverters, the fundamental operation of a battery is inherently DC-based. Understanding this basic principle is essential for anyone working with or interested in electronics and energy storage.
