Why Is A Catalytic Converter So Expensive
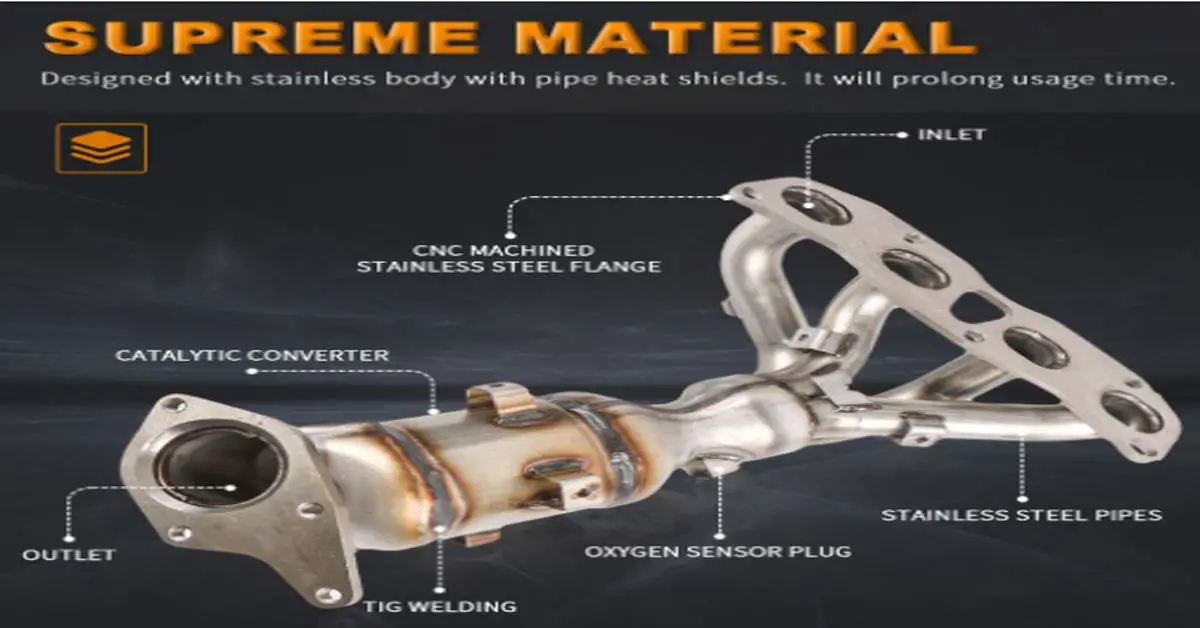
So, you're looking at replacing your catalytic converter and you've choked on the price tag. You're not alone. It's a common reaction, and understanding why they're so expensive involves delving into their design, the materials used, and the market forces at play. Let's break it down.
The Job of a Catalytic Converter: A Chemistry Lesson
First, a quick review of what a catalytic converter does. Its primary function is to reduce harmful emissions from your engine's exhaust. We're talking about pollutants like:
- Hydrocarbons (HC): Unburned fuel, a significant contributor to smog.
- Carbon Monoxide (CO): A poisonous, odorless gas.
- Nitrogen Oxides (NOx): These contribute to acid rain and respiratory problems.
The converter achieves this through a process called catalysis. It doesn't "burn" the pollutants; instead, it provides a surface where chemical reactions can occur more efficiently. This is where the "catalytic" part comes in. The converter uses precious metals to accelerate these reactions.
There are two main types of reactions happening inside a typical three-way catalytic converter (the most common type since the 1980s):
- Reduction of NOx: NOx molecules are broken down into nitrogen (N2) and oxygen (O2).
- Oxidation of HC and CO: HC and CO are combined with oxygen (O2) to form carbon dioxide (CO2) and water (H2O).
So, the nasty stuff goes in, and relatively harmless stuff (relatively!) comes out.
The Precious Metals: The Heart of the Cost
Now, for the core reason behind the high price: precious metals. Catalytic converters rely heavily on platinum, palladium, and rhodium. These metals are extremely effective catalysts for the reactions we discussed. They provide the necessary surface area and chemical properties to facilitate those reactions at the exhaust temperatures typically seen in modern engines.
Here's a breakdown of each metal's role:
Platinum (Pt)
Platinum is primarily used for the oxidation of hydrocarbons (HC) and carbon monoxide (CO). It's highly effective at promoting the conversion of these pollutants into less harmful substances.
Palladium (Pd)
Palladium also contributes to the oxidation of HC and CO. In some converters, particularly those designed for lean-burn engines, palladium might be the primary catalyst for oxidation.
Rhodium (Rh)
Rhodium is the key catalyst for the reduction of nitrogen oxides (NOx). It's far more effective than platinum or palladium at breaking down NOx into nitrogen and oxygen.
Why these metals? Their unique electronic configurations and chemical inertness at high temperatures make them ideal catalysts. They can withstand the harsh conditions within the exhaust system and maintain their catalytic activity over extended periods.
However, these metals are rare and expensive. Their price fluctuates based on supply, demand, geopolitical factors, and speculation in the commodities market. Significant increases in the price of these metals directly translate to a higher cost for catalytic converters.
To illustrate, consider the following (hypothetical) example. Let's say a converter contains:
- 3 grams of platinum
- 1 gram of palladium
- 0.5 grams of rhodium
If platinum is $1000/gram, palladium is $2000/gram, and rhodium is $5000/gram, the metal cost alone is (3 * $1000) + (1 * $2000) + (0.5 * $5000) = $3000 + $2000 + $2500 = $7500. Of course, these are just example figures, and the actual quantities and prices vary, but this illustrates the significant impact the metals have on the overall cost.
The Construction and Complexity
It's not just the metals themselves; the way they're incorporated into the converter adds to the cost. The precious metals aren't used in their pure form. Instead, they're typically dispersed as tiny particles (nanoparticles) on a high-surface-area substrate.
This substrate is usually a ceramic honeycomb structure. The honeycomb design maximizes the surface area exposed to the exhaust gases, improving catalytic efficiency. The precious metals are then applied to this honeycomb as a thin coating, often through a process called washcoating.
The washcoat material itself is typically a metal oxide, such as alumina (aluminum oxide), which further increases the surface area and provides a stable support for the precious metal nanoparticles.
Manufacturing this complex structure requires specialized equipment and precise control over the coating process. Any defects or inconsistencies in the coating can reduce the converter's efficiency and lifespan.
Furthermore, the catalytic converter needs a robust housing to protect the ceramic core from damage. This housing is usually made of stainless steel, which is resistant to corrosion and high temperatures. The welding and assembly of this housing also add to the manufacturing cost.
Regulations and Testing
Strict emission regulations in many countries mandate the use of catalytic converters. Manufacturers must meet specific performance standards, which require rigorous testing and certification. This testing adds to the overall cost of developing and producing catalytic converters.
In the United States, the Environmental Protection Agency (EPA) sets emission standards and oversees the testing and certification of catalytic converters. Converters must meet stringent requirements for reducing emissions of HC, CO, and NOx. Furthermore, they must maintain their effectiveness for a specified mileage (e.g., 100,000 miles) to comply with warranty regulations.
Theft and Recycling
Sadly, the high value of the precious metals has made catalytic converters a frequent target for theft. Thieves often steal converters to extract the metals and sell them for scrap. This increases the demand for replacement converters, further driving up prices.
On a more positive note, catalytic converters can be recycled. Recycling companies recover the precious metals from spent converters, reducing the need for new mining and potentially lowering the long-term cost of these materials. However, the recycling process is complex and requires specialized equipment and expertise.
Aftermarket vs. OEM
When replacing your catalytic converter, you'll likely encounter the option of aftermarket converters. These are generally cheaper than Original Equipment Manufacturer (OEM) parts, but there are tradeoffs.
OEM converters are designed and tested to meet the exact specifications of your vehicle. They're typically more expensive but offer guaranteed performance and durability. Aftermarket converters, on the other hand, may use less expensive materials or have a different design. While some aftermarket converters perform well, others may not meet emission standards or may have a shorter lifespan.
Choosing between aftermarket and OEM depends on your budget and priorities. If you're concerned about emissions compliance and long-term reliability, an OEM converter is generally the better choice. However, if you're on a tight budget, a reputable aftermarket converter might be a viable option, but be sure to research its performance and warranty before buying.
In Conclusion
The high cost of catalytic converters is a complex issue driven by the rarity and expense of precious metals, the sophisticated manufacturing process, strict emission regulations, and even theft. While alternative technologies are being explored, catalytic converters remain essential for controlling emissions and protecting air quality. Understanding these factors can help you make informed decisions when replacing your converter and appreciate the technology behind this vital component of your vehicle.
