How Does A Battery Work In A Car
Alright, let's dive into the heart of your car's electrical system – the battery. Understanding how your car battery works isn't just about impressing your friends; it's crucial for troubleshooting electrical issues, performing basic maintenance, and even knowing when it's time for a replacement. Think of this as your guide to battery internals. We've got a handy diagram you can download (more on that later), and this article will help you make sense of every line, symbol, and component within it.
Purpose: Why This Knowledge Matters
Why bother understanding your car battery? Well, beyond basic car care, knowing the inner workings of your battery empowers you to:
- Diagnose starting problems: Is it the starter motor, the solenoid, or a dead battery? Understanding the battery's role will help you pinpoint the culprit.
- Perform routine maintenance: Cleaning terminals, checking electrolyte levels (in older batteries), and testing the battery's voltage are all easier when you understand what's going on inside.
- Choose the right replacement: Knowing your car's specific battery requirements (size, CCA, reserve capacity) helps you select the correct battery.
- Avoid costly mistakes: Incorrect jump-starting, overcharging, or neglecting a failing battery can damage your electrical system.
- Customize your electrical system: Planning to add auxiliary lights, a powerful audio system, or other electrical accessories? You'll need to understand your battery's capacity and limitations.
Key Specs and Main Parts
Before we get to the nitty-gritty, let's cover the basics. A car battery is a rechargeable lead-acid battery designed to provide a large surge of electrical current to start the engine. Key specifications include:
- Voltage (V): Typically 12V in most passenger vehicles. This is the electrical potential difference the battery provides.
- Cold Cranking Amps (CCA): A measure of the battery's ability to start an engine in cold temperatures. Higher CCA is generally better, especially in colder climates.
- Amp-Hours (Ah): Represents the battery's capacity to deliver a certain amount of current over a specific period. A higher Ah rating means the battery can power accessories for longer.
- Reserve Capacity (RC): The amount of time (in minutes) a fully charged battery can deliver 25 amps before dropping to 10.5 volts. This is important if your alternator fails while driving.
The main parts inside a typical lead-acid car battery are:
- Positive Plates: Composed of lead dioxide (PbO2).
- Negative Plates: Composed of pure lead (Pb).
- Electrolyte: A solution of sulfuric acid (H2SO4) and water.
- Separators: Porous insulators that prevent the positive and negative plates from touching and short-circuiting.
- Cells: Six individual cells, each producing approximately 2.1 volts, connected in series to achieve the 12-volt output.
- Terminals: The positive (+) and negative (-) posts to which the vehicle's electrical system connects.
- Case: A durable enclosure that contains all the components and prevents leaks.
Symbols: Deciphering the Diagram
Our downloadable diagram uses standard electrical symbols to represent the components and connections within the battery. Here's a breakdown of some common symbols:
- Solid Lines: Represent electrical conductors, usually wires or metal straps.
- Dashed Lines: May indicate internal connections or the flow of ions within the electrolyte.
- "+" Symbol: Indicates the positive terminal of the battery.
- "-" Symbol: Indicates the negative terminal of the battery.
- Rectangles: Often represent individual battery cells.
- Labels (e.g., PbO2, Pb, H2SO4): Identify the chemical composition of the various components.
- Arrows: May indicate the direction of current flow or ion movement.
- Colors: Typically, red is used for positive connections and black for negative connections. Blue may be used for other specific connections.
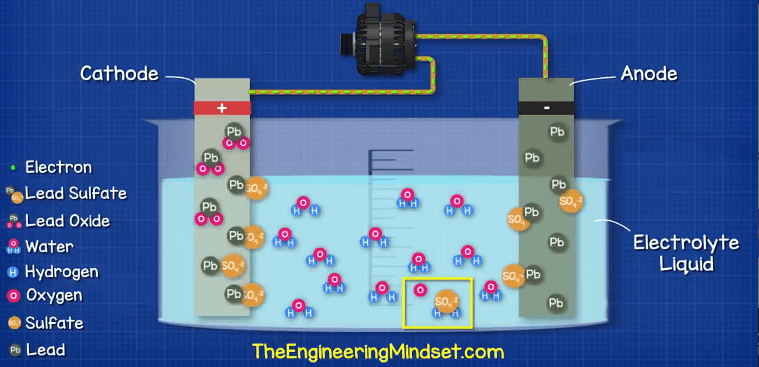
How It Works: A Chemical Symphony
The heart of the car battery's operation lies in a chemical reaction between the lead plates and the sulfuric acid electrolyte. Here's the breakdown:
Discharging (Supplying Power):
When you turn the ignition key, a circuit is completed, allowing current to flow from the battery to the starter motor. This triggers a chemical reaction called oxidation-reduction (redox). At the negative plate (lead), lead atoms (Pb) lose electrons and become lead ions (Pb2+). These ions react with sulfuric acid (H2SO4) to form lead sulfate (PbSO4) and release electrons. At the positive plate (lead dioxide), lead dioxide (PbO2) reacts with sulfuric acid and electrons from the external circuit to also form lead sulfate (PbSO4) and water (H2O). The flow of electrons through the external circuit is what provides the electrical current to power your car's systems. Note that both the positive and negative plates are gradually converted into lead sulfate during discharge. The sulfuric acid concentration in the electrolyte decreases as it is consumed in the reaction.
Charging (Recharging the Battery):
When the engine is running, the alternator supplies electricity to recharge the battery. This reverses the chemical reaction described above. Electrons are forced back into the negative plate, converting the lead sulfate (PbSO4) back into lead (Pb). At the positive plate, electrons are drawn from the lead sulfate (PbSO4), converting it back into lead dioxide (PbO2). The sulfuric acid (H2SO4) is also regenerated, increasing the electrolyte concentration. This process effectively restores the battery to its fully charged state.
Cell Voltage: Each of the six cells in a 12-volt battery produces approximately 2.1 volts due to the chemical reactions. Connecting these cells in series allows their voltages to be added together, resulting in the total 12-volt output.
Real-World Use: Basic Troubleshooting Tips
Knowing how your battery works can help you troubleshoot common issues:
- Slow Cranking: Could indicate a low battery charge, corroded terminals, or a failing starter motor. Clean the terminals and check the battery voltage with a multimeter. A fully charged 12V battery should read around 12.6 volts or higher.
- Battery Won't Hold a Charge: Could be due to a parasitic drain (something drawing power when the car is off), a faulty alternator, or a damaged battery. Have the battery load-tested by a professional to determine its condition.
- Corrosion on Terminals: Clean the terminals with a wire brush and a solution of baking soda and water. Apply a corrosion inhibitor to prevent future buildup.
- Bulging Battery Case: Indicates overcharging, excessive heat, or internal damage. Replace the battery immediately.
Safety: Proceed with Caution
Working with car batteries involves some risks, so always take precautions:
- Sulfuric Acid: The electrolyte is highly corrosive and can cause severe burns. Wear eye protection and gloves when handling batteries. In case of contact, flush immediately with plenty of water.
- Hydrogen Gas: Batteries release hydrogen gas during charging, which is highly flammable and potentially explosive. Avoid sparks or open flames near batteries. Ensure adequate ventilation when charging.
- Short Circuits: Avoid accidentally short-circuiting the battery terminals. This can generate a large amount of heat and cause damage or injury. Always disconnect the negative terminal first when working on the electrical system.
- Battery Weight: Car batteries are heavy and can be awkward to lift. Use proper lifting techniques to avoid back injuries.
Risky Components
Specifically, be mindful of the following:
Electrolyte: Handle with extreme care due to its corrosive nature.
Terminals: Avoid short-circuiting them to prevent sparks and potential burns.
Internal Components (plates, separators): Do not attempt to disassemble the battery yourself. This can expose you to hazardous materials and potentially lead to injury.
We have the detailed battery diagram file ready for you to download. Having this at hand as you troubleshoot or learn more about your car will be very useful.
