What Is The Charge Of Strontium
Alright, let's talk about strontium and its charge. Now, why are we discussing this in the context of cars, modding, and DIY mechanics? Because understanding the fundamental building blocks of matter, like knowing the charge of strontium (Sr), can be surprisingly useful. Think of it like knowing the different metal alloys in your engine block – you don't need to know the exact atomic weight *every* time, but having a foundational understanding of material properties helps you diagnose issues, understand how different components interact, and even predict potential problems during modifications.
Purpose: Why Strontium Matters (Even in Cars!)
While you won't find pure strontium used directly in most car components, understanding its behavior helps you grasp broader chemical concepts. For example, knowing about ionic bonding, which strontium readily participates in, is vital for understanding how battery electrolytes work, how corrosion occurs (and how to prevent it), and even how catalytic converters reduce emissions. When we talk about the charge of an element, we're getting to the heart of its chemical personality – how it interacts with other elements to form compounds. This understanding is crucial for tackling tasks like choosing the right anti-seize compound, understanding the long-term effects of different fuel additives, or even safely handling battery acid.
Key Specs and Main Parts: Atomic Structure Essentials
To understand the charge of strontium, we need a quick refresher on atomic structure. An atom is made up of three main particles:
- Protons: Positively charged particles located in the nucleus (the atom's center).
- Neutrons: Neutral (no charge) particles also located in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells.
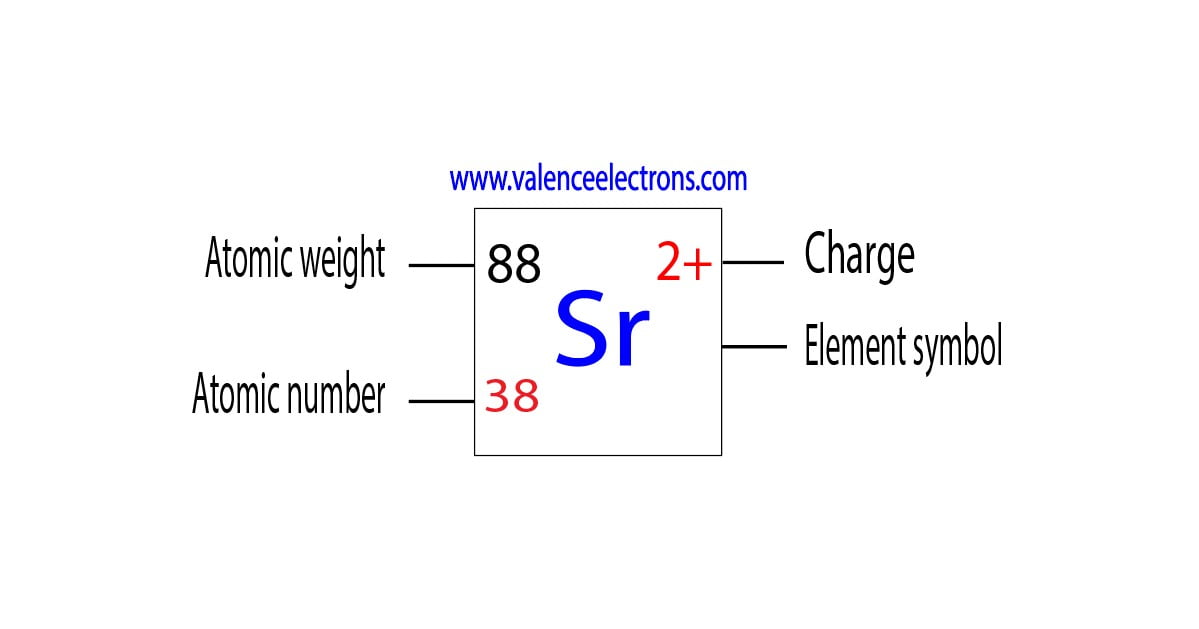
An atom is electrically neutral when the number of protons equals the number of electrons. The atomic number of an element is the number of protons in its nucleus. Strontium (Sr) has an atomic number of 38, meaning it has 38 protons. Now, here's the kicker: atoms *want* to achieve a stable electron configuration, which usually means having a full outermost electron shell. This is where the concept of ions and charge comes in.
The number of electrons in an atom's outer shell dictates its reactivity. This is determined by the element’s position in the periodic table. Elements in Group 1 (like sodium and potassium) have one electron in their outer shell. Elements in Group 2 (like strontium, magnesium, and calcium) have two.
Charge Explained: Strontium's +2 Oxidation State
Strontium is in Group 2 of the periodic table, also known as the alkaline earth metals. These elements have two valence electrons – that is, two electrons in their outermost shell. To achieve a stable configuration, strontium readily *loses* these two electrons. When it loses these two negatively charged electrons, it becomes an ion with a net positive charge. This net positive charge is equal to the number of electrons lost. Therefore, strontium forms a +2 ion (Sr2+). We say that strontium has an oxidation state of +2 when it's in a compound. Oxidation state is a way to track how many electrons an atom has gained or lost when bonding.
How It Works: The Ionic Bond Formation
The +2 charge of strontium is crucial in forming ionic bonds. Ionic bonds occur when electrons are transferred between atoms, creating ions (atoms with a charge). Since strontium has a +2 charge after losing its two electrons, it's attracted to negatively charged ions (anions). For example, strontium can react with oxygen (O), which needs to gain two electrons to complete its outer shell. Oxygen forms an O2- ion. The Sr2+ and O2- ions are strongly attracted to each other, forming strontium oxide (SrO), an ionic compound.
This electron transfer isn't a one-way street, more like a give-and-take that results in stability for both involved atoms. The electrostatic attraction between the positive strontium ion and the negative oxygen ion is what holds the strontium oxide molecule together.
Real-World Use: Troubleshooting and Understanding Material Properties
Okay, back to the garage. While you likely won't be directly working with elemental strontium, understanding its behavior as a +2 ion helps you in several ways:
- Corrosion Prevention: Knowing that metals like to lose electrons (oxidize) helps you understand why corrosion occurs and how protective coatings work. Coatings are used to create a barrier between the metal and the environment, preventing the transfer of electrons that leads to rust.
- Battery Electrolytes: Battery electrolytes contain ions that conduct electricity. Understanding ionic charges helps you comprehend how batteries function and why certain materials are suitable for electrolytes.
- Catalytic Converters: Catalytic converters use metals like platinum and palladium to catalyze chemical reactions that reduce harmful emissions. Understanding the oxidation states of these metals is key to understanding how the converter works.
- Choosing the Right Grease: Certain greases are designed to prevent galvanic corrosion, which occurs when two dissimilar metals are in contact in the presence of an electrolyte. The electrochemical properties of the metals (their tendency to lose electrons) are crucial in understanding and preventing this type of corrosion.
Basic Troubleshooting Tip: If you're dealing with corrosion, consider the electrochemical properties of the metals involved. Are they likely to form ions easily? Is there an electrolyte present (water, salt, etc.)? Understanding these factors can help you choose the right cleaning products and protective measures.
Safety: Handling Ionic Compounds
While elemental strontium is reactive with air and water, you're more likely to encounter it in compound form. While many strontium compounds are relatively safe, some can be toxic. Always consult the Material Safety Data Sheet (MSDS) or Safety Data Sheet (SDS) for any chemical you're working with. The SDS will provide information on potential hazards, handling precautions, and first aid measures. When dealing with chemicals, always wear appropriate personal protective equipment (PPE), such as gloves, eye protection, and a respirator if necessary.
Risky Components: When it comes to car-related applications, batteries contain sulfuric acid, a highly corrosive electrolyte. This electrolyte dissociates into ions, and understanding the charges of these ions is vital for comprehending the battery's function and the dangers of mishandling battery acid.
Conclusion
So, there you have it: Strontium has a +2 charge because it readily loses two electrons to achieve a stable electron configuration. This seemingly simple fact has far-reaching implications, from understanding corrosion to comprehending how batteries and catalytic converters function. While you might not use this knowledge every day, having a basic understanding of atomic structure and ionic bonding will make you a more informed and capable DIY mechanic.
We have a detailed diagram illustrating the electronic configuration and ionization process of strontium available for download. This diagram provides a visual representation of the concepts discussed in this article and can be a valuable tool for further learning. Feel free to reach out for the file!
